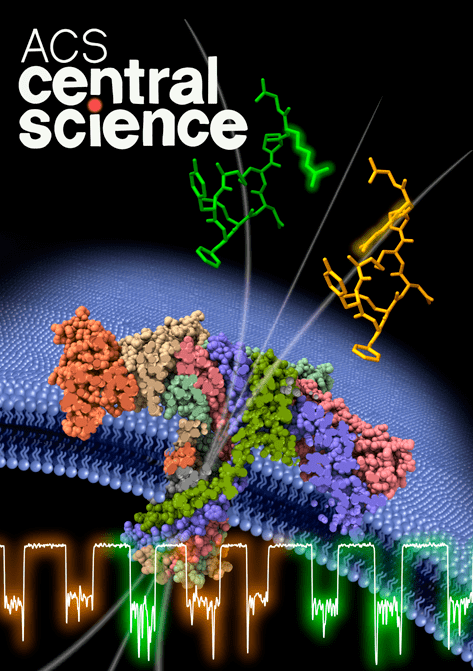
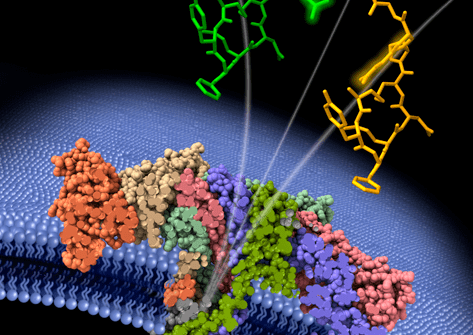
A team of researchers from Lambe’s Évry, and Cergy sites have shown that aerolysin nanopores can be used to discriminate medically pertinent peptides differing only in the “handedness” (called enantiomers) of an included amino acid. That conformational change can result in disease, or, quite the contrary, provide therapeutic properties. With three publications in only two years, the team has become a world leader in the detection of peptide biomarkers of great importance in medical diagnostics.
The spatial conformation of proteins and peptides is vital for their biological activities and largely determined by their constituent amino acids. However, research has shown that peptides sharing a same amino acid sequence can differ in activity, sometimes with pathological consequences, according to the orientation of one of those constituents. Indeed, all chiral amino acids have two conformations, as if reflecting one another in a mirror. “Chiral” comes from the ancient Greek word for hand, and indeed one’s hands placed side-by-side are a good metaphor for chirality. Each conformation is called an enantiomer. the “L” (from the Latin laevus for “left”) conformation is the most frequent, and the “D” (Latin dexter for “right”) version, its reflection, more rare. That difference cannot be detected by conventional techniques, such as high-performance liquid chromatography (HPLC) or mass spectrometry. And yet, it is of great medical interest.
Professor Juan Pelta’s Genopole team at Lambe (Analysis, Modeling and Materials for Biology and the Environment), created in 2007 thanks to Genopole’s Atige program*, has developed a technique able to discriminate specific enantiomers in a model peptide, and even particular states of this latter. To do so, they applied a unique molecule detection system involving a wild-type aerolysin nanopore that they have been studying for more than 12 years now. That system detects the specific electrical signatures of biomolecules as they pass, one after the other, through the aerolysin nanopore (see below, “A few reminders on the aerolysin nanopore method”).
In 2023, the team demonstrated the nanopore’s potential for detecting coagulation biomarkers, and in January 2024, its ability to distinguish and quantify, directly in human serum, two pathological kinins differing by a single amino acid article in French). With their latest work published in May 2024 in ACS Central Science—and making its cover (image above)—the Lambe team has demonstrated an even higher level of precision for their system. That May 2024 article also makes the Genopole team the leader in published articles on peptide markers over the last few years.
The nanopore method detects and quantifies the L and D forms of vasopressin
In their most recent work, the researchers’ model molecule was arginine vasopressin (L-Arg-AVP), a peptide comprising nine amino acids. L-Arg-AVP is produced in the hypothalamus and involved in water balance & blood pressure regulation, and many other aspects of life as well (social behavior, memory, cardiovascular health, etc.). Particularly, it is a marker of diabetes.
L-Arg-AVP has a loop structure resulting from disulfide bridges between two cysteines, then a terminus counting 3 amino acids, including one L-arginine. The D-Arg-AVP enantiomer is synthesized for research and therapeutic purposes.
With the aerolysin nanopore method, either L- or D-Arg-AVP-containing solutions produce partially overlapping but nonetheless distinguishable electronic signatures at an optimal voltage of 110 mV (Figure 1). By submitting data from the molecule’s passage through the nanopore (minimum/maximum electrical blockades, dwell times, etc.) to principal component analysis (a methodology for statistical analyses), the researchers were able to reach a 99% identification rate for the L and D forms.

The signatures express the level of current blockade measured during the molecule’s passage through the nanopore and the number of passages for each level.
Thereafter, the team tested both equimolar (1:1) and nonequimolar (1:3 and 3:1) mixes of the two conformations. Differentiating the L and D conformation signals was difficult with a simple visual analysis (Figure 1). However, the team was able to do so using principal component analysis in association with Monte Carlo prediction (a repeated random sampling approach) (Figure 2). For example, a success rate of 73% was reached for the equimolar mix. The remaining, nonidentified 27% was attributed to the partial overlapping of the L and D signatures.

The signal is attributed to the L (green) or D (orange) conformation of vasopressin (blue: signal non-attributable to L or D).
The correlation established between the real and predicted ratio demonstrates the method’s quantitative potential.
Moreover, the team’s approach furnished a very good correlation between the predicted and real proportions in the mix (Figure 2), demonstrating the approach’s potential for quantitative diagnostics involving peptide biomarkers. And finally, the researchers were also able to detect vasopressin’s “open” and “saddle” conformations (which occur due to different bonds within the molecule) and replicate known proportions of those 3D conformations.
A few reminders on the aerolysin nanopore method
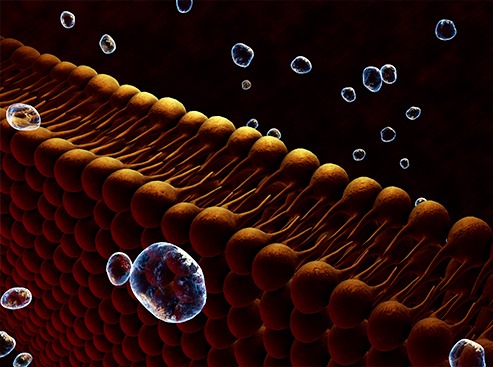
 Aerolysin forms nanometric pores (<2 nm in diameter) in cell membranes. The Évry/Cergy research team uses that aerolysin property to create nanopores in a lipid membrane separating two compartments filled with electrolyte solution. When subjected to an electrical field, the molecules in one compartment are drawn to and traverse the nanopore, one by one, to the other compartment. In so doing, each molecule partially and temporarily blocks the ionic current (see illustration). A molecule going through the nanopore will show a particular current blockade intensity and dwell time determined by its size, shape, and charge. In this way, each molecule creates a specific electrical signature.
Aerolysin forms nanometric pores (<2 nm in diameter) in cell membranes. The Évry/Cergy research team uses that aerolysin property to create nanopores in a lipid membrane separating two compartments filled with electrolyte solution. When subjected to an electrical field, the molecules in one compartment are drawn to and traverse the nanopore, one by one, to the other compartment. In so doing, each molecule partially and temporarily blocks the ionic current (see illustration). A molecule going through the nanopore will show a particular current blockade intensity and dwell time determined by its size, shape, and charge. In this way, each molecule creates a specific electrical signature.
Towards precision diagnostics
The Lambe team has again advanced its demonstration of the potential of its nanopore system for identifying specific peptides with complex, three-dimensional structures. They have shown that it is able to distinguish even very similar conformations and quantify their proportions in a mix.
The current medical literature shows a growing interest for peptide biomarkers. Being able to detect their sequential and conformational variations is also an issue in health. The work done by the Genopole team shows the scope of pathologies associated with peptide biomarkers. Over their three most recent publications, the team has worked with blood coagulation-associated peptides involved in strokes, heart attacks and cancer, kinins involved in angioedema, and an antidiuretic hormone involved in diabetes.