Researchers from Genopole’s Lambe Lab the Lariboisière Hospital and the start-up Dreampore joined forces to demonstrate the ability of a natural nanopore to detect coagulation biomarkers associated with strokes, heart attacks and cancers. Their work, published 3 February 2023 in ACS Central Science, lays a path toward new, rapid and discriminating medical diagnostics.
 One of the major challenges facing our world, where the human population is both growing and aging, is the early and exact diagnosis of serious diseases to enable prevention and personalized treatments. There is thus a need to develop highly sensitive biomarker detection tests deployable in easily accessed biological fluids like blood or urine. To address that need, researchers from Genopole’s Laboratory for Analysis and Modeling in Biology and the Environment (Lambe – University of Évry/University of Cergy-Pontoise/CNRS) teamed with colleagues from Lariboisière Hospital’s Center for Biological Resources and the start-up Dreampore to strong>demonstrate proof of concept for a novel approach involving the electronic detection of biomarkers via a natural nanopore.
One of the major challenges facing our world, where the human population is both growing and aging, is the early and exact diagnosis of serious diseases to enable prevention and personalized treatments. There is thus a need to develop highly sensitive biomarker detection tests deployable in easily accessed biological fluids like blood or urine. To address that need, researchers from Genopole’s Laboratory for Analysis and Modeling in Biology and the Environment (Lambe – University of Évry/University of Cergy-Pontoise/CNRS) teamed with colleagues from Lariboisière Hospital’s Center for Biological Resources and the start-up Dreampore to strong>demonstrate proof of concept for a novel approach involving the electronic detection of biomarkers via a natural nanopore.
For their study, the team set its sights on a medically important family of biomarkers: those involved in coagulation. The researchers looked specifically at fibrinopeptide A (FPA), a coagulation disorder marker detectable in blood and urine, and associated with strokes, heart attacks and cancers. However, FPA exists in native, modified (comprising post-translational phosphorylation; denoted FPA-P) and cleaved forms. The FPA assays that are currently available differentiate these forms poorly, and moreover, they are very time-consuming. Thus, this marker is underemployed in routine clinical practice.
A specific electrical signal when the biomarker passes through the nanopore
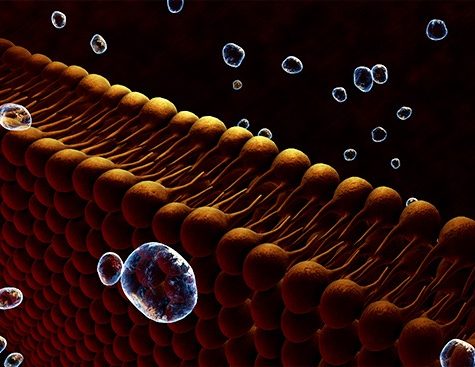
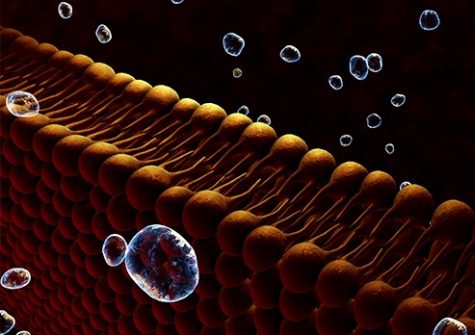
Laureate of Genopole’s 2007 Atige* program, Juan Pelta, , and his team have developed a technology to identify amino acids and short chains of these latter called peptides (Cressiot et al., Small Methods, 2020 https://doi.org/10.1002/smtd.202000090). Their approach calls upon a property of a natural molecule called aerolysin (Cressiot et al., ACS sensors, 2019 https://doi.org/10.1021/acssensors.8b01636), to form nanometric pores in cell membranes. They used that aerolysin property to create nanopores in an otherwise impermeable lipid membrane separating two solution-filled compartments. When subjected to an electrical field, the molecules in one compartment are drawn to and traverse the nanopore, one by one, to the other compartment. In so doing, each molecule partially and temporarily blocks the ionic current. The resulting electrical signal is specific to the molecule’s shape, size and chemical modification.
That technology applied to FPA in the team’s study allowed the researchers to detect and identify the peptide biomarker and its derivatives present in a solution. They showed that FPA, FPA-P and the smaller cleaved forms could be differentiated by two parameters of each form’s unique electrical signal: dwell time (the duration of the peptide’s immobilization in the nanopore) and blockade level (how much the peptide’s passing blocked the electrical current) (see graphical abstract ⬇️).
A promising method for medical diagnostics
The system of measuring an electrical current through an aerolysin nanopore developed by the Genopole and Cergy Pontoise researchers provides new, promising vistas for precision diagnostics. There remain two obstacles to overcome. The first is the ability to detect very low concentrations in biological fluids for early diagnoses. This may involve, for example, modifying the nanopores to increase their attraction force (pressure and voltage) and thus increase the likelihood that the biomarker passes through, even at very low concentrations. The second obstacle involves successfully identifying the biomarker of interest in complex fluids like urine and blood, both containing numerous other small molecules.

➡️ To learn more about the nanopore technology and the team’s 2020 work