The promising results find their roots notably in the work of Nicolas Tricaud, who, in 2020, created the Diseases of Myelinated Nerve Fibers research team at I-Stem thanks to an Atige grant from Genopole.
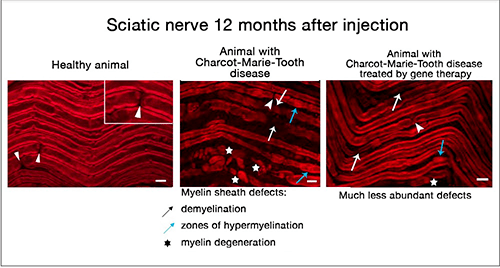
Charcot-Marie-Tooth (CMT) disease is a multiform genetic pathology that causes limb-extremity muscle atrophy, hand and foot deformations and associated mobility handicaps. CMT disease type 1a is caused by the duplication of the gene coding for the protein PMP22. The resulting overexpression of this latter hampers the correct formation of the myelin sheath responsible for good nerve conduction.
The gene therapy approach developed by the team’s researchers employs an RNA interference approach to treat Schwann cells affected by the disease. Specifically, they used a particular type of RNA, called a small hairpin inhibitory RNA (shRNA), to shackle the messenger RNA carrying the code for the PMP22 protein. An adeno-associated viral vector type 9 carrying the shRNA and targeted to the Schwann cells was injected directly into the sciatic nerves of the rats affected by the disease.
A single injection at a young age restored close-to-normal PMP22 expression levels and prevented disease-associated motor and sensory deficits for one year, i.e., approximately a third of the rat’s normal lifespan. The team’s study was published in Nature Communications on 21 April 2021.
The direct intraneural injection was successful in delivering the vector and its therapeutic molecule to a large proportion of the targeted Schwann cells.
That approach also avoided the immune response, which can greatly reduce the efficacy of treatments delivered by viral vectors injected systemically.