Two of Genopole’s key actors in innovative biotherapies, the laboratory Genethon and the gene therapy production facility Yposkesi, contributed to this success.
X-linked chronic granulomatous disease is a rare and severe immune disorder affecting only males and usually in childhood. The gene therapy* developed at Genethon by Dr. Anne Galy is the first to offer durable treatment for the disease. Genethon developed the delivery vector for the therapy and piloted the initial European trials launched in 2013. The results from Europe and the United States were published in the 28 January 2020 edition of Nature Medicine.
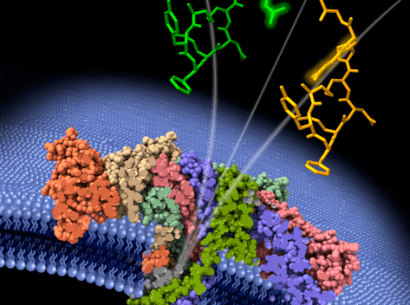
The gene therapy is designed to restore the activity of a faulty enzyme, NAPDH oxidase, by inserting a functional gene for that enzyme in the patient’s immune cells. To deliver the genes to those cells, the therapy uses a lentiviral vector that has already demonstrated its efficacy and safety in other immunodeficiencies.
The clinical vector lots were produced by Yposkesi, France’s first nonprofit pharmaceutical establishment created by AFM-Téléthon and Bpifrance.
Today, gene therapy research is bringing a first wave of successful treatments not only for previously incurable rare genetic diseases, including immunodeficiencies, blood diseases, vision disorders and recently a myopathy, but also for several frequent pathologies, like leukemia.