Their objective is to provoke the expression of different therapeutic proteins in the blood and thus enable treatments for genetic blood diseases such as hemophilia, or certain metabolic disorders.
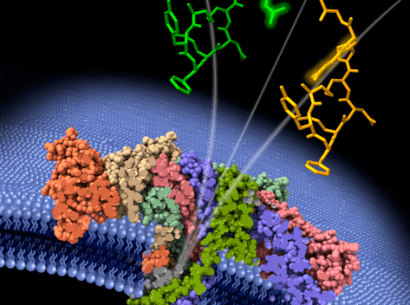
The platform developed by the Integrare team uses CRISPR-Cas9 gene editing technology. Their approach involves modifying hematopoietic stem cells (HSCs) ex vivo to obtain the expression of therapeutic proteins in the erythroid lineage, i.e., the cell line resulting in red blood cells. Thanks to the precision of the CRISPR-Cas9 “molecular scissors”, the team was able to place the genes of therapeutic interest under the control of the highly-active and erythroid-specific α-globin gene promoter naturally present in HSCs.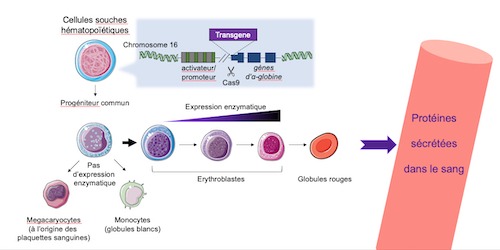
The team’s approach combined the advantages of maximizing the quantity of therapeutic proteins produced and placing that production in the most populous cells resulting from hematopoiesis, that is, those involved in and resulting from erythropoiesis.
Using that technique, the researchers showed that the modified erythroblasts expressed and secreted the therapeutic proteins and that these latter conserved their enzymatic activity. Ultimately secreted into the blood, those proteins are captured by the pathological cells wherein they are able to correct specific disorders.
The Integrare team’s platform can be used to treat not only a number of human genetic pathologies, for example hemophilia or certain metabolic disorders, but also a number of non-genetic illnesses as well.