Virginie Mournetas* and the Muscular Diseases team headed by Christian Pinset at I-Stem (Inserm UMR 861, AFM-Téléthon) have shown that molecular dysregulations associated with Duchenne muscular dystrophy appear during embryonic development before muscle tissues even start to develop. The combination of an in vitro cellular model of muscle formation created from pluripotent stem cells and a multiomic approach enabled this discovery.
Duchenne muscular dystrophy (DMD) is a rare genetic disease occurring in roughly 1 out of 5000 male births and causing progressive muscle degeneration. The disease is usually diagnosed around the age of four, when muscle damage has already become significant. The efficacy of certain treatments may be hampered by this belated diagnosis, which is particularly regrettable given that current findings, some born of research at I-Stem, suggest that the disease may appear before birth. Indeed, one of the laboratory’s objectives is to better identify the moment at which the disease begins to manifest.
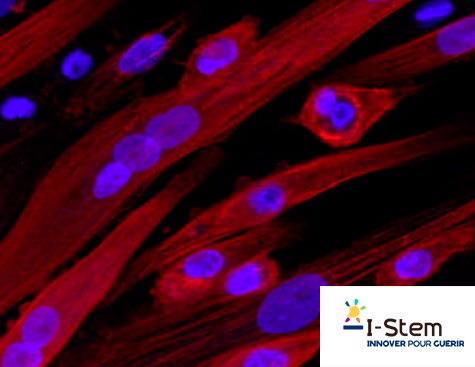
The I-Stem Muscular Diseases team designed an original study on DMD onset involving a protocol to guide iPS cell differentiation toward the muscle cell phenotype. Their protocol faithfully reproduced the key steps in myogenesis, (i.e., the formation of skeletal muscle during embryonic development): first the formation of the mesoderm, then the somites (an intermediate structure) and through to the development of myotubes (see photo), a precursor to muscle fibers. They used the protocol on iPS cells derived from either healthy people or diseased patients. Multiomics analyses (transcriptomics, microRNA, proteomics) were done at five time intervals during the 25 days of myotube differentiation to determine molecular activity in the healthy or pathological cells.
As early as the somite stage, 10% of the pathological transcriptomes (indicating the genes expressed by the cells) showed dysregulations compared to the healthy transcriptomes. Notably, those dysregulations concerned mitochondrial genes, known for their role in DMD.
This unprecedented study demonstrating a very early manifestation of DMD during embryonic development and even upstream of muscle formation itself suggests a need for a reappraisal of the disease’s natural history. Particularly, the defective dystrophin protein, which is highly deleterious to muscle fibers and the cause of DMD, could also have other effects. This aspect merits exploration as it may explain some non-muscular DMD symptoms. Toward that goal, all of the study’s analyses have been grouped in a database available to the scientific community.