Xavier Nissan’s muscular dystrophies pharmacology team at I-Stem, in partnership with Isabelle Richard from Généthon and the company Kantify, has identified a pharmacological combination conferring a novel mode of action potentially efficacious against not only limb-girdle muscular dystrophy types R3s and R5 but also cystic fibrosis. The discovery was made possible by marrying competencies and knowledge in cellular models for disease, high throughput drug screening, myopathy mechanisms and artificial intelligence.
Limb-girdle muscular dystrophy (LGMD) type R3, like the 30 some-odd other types of the disease, presents as progressive weakening of mostly the hip and shoulder muscles. This particular type is caused by mutations in alpha-sarcoglycan, a protein within the dystrophin glycoprotein complex, itself an element within the membrane of striated muscle that protects this latter from contraction-induced damage. The mutation most frequently associated with the disease, called R77C, involves an amino acid substitution resulting in abnormal folding of α-sarcoglycan. Once identified by a cellular quality control mechanism, the misfolded protein is broken down by proteasomes, one of the cell’s many organelles.
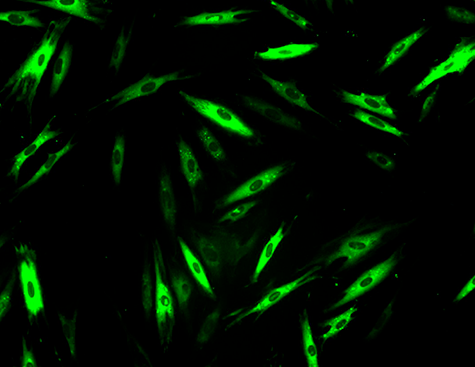
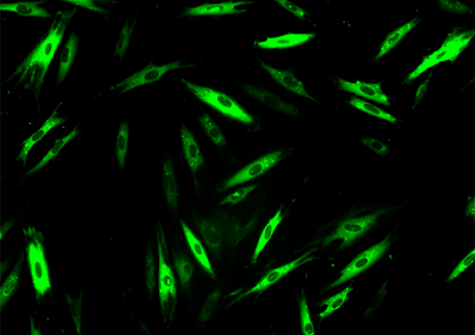
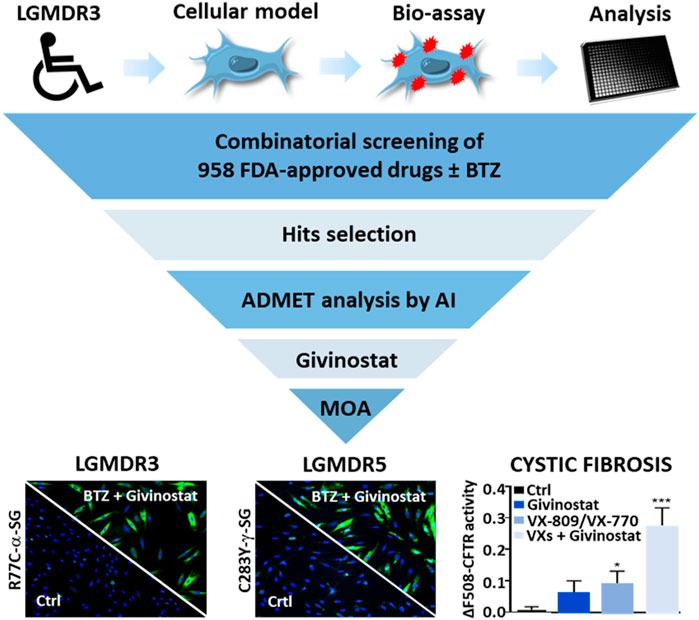
Researchers from two of Genopole’s laboratories, I-Stem and Genethon (AFM-Téléthon, Inserm, University of Evry Paris-Saclay) teamed to identify drugs capable of inhibiting that breakdown, because even misfolded, α-sarcoglycan does remain partially functional. They thus developed a cellular model of the disease from patient fibroblasts and thereafter tested 958 drugs already approved by the American Food and Drug Administration for other indications. This exploration of drugs approved for other uses, called drug repositioning, offers many advantages, including the possibility of getting treatments to patients faster. The pharmaceutical compounds were tested alone or in combination with a low dose of bortezomib, a molecule known to inhibit the proteasome but also associated with cytotoxic effects at efficacious doses. Thus, the objective of the team’s approach was to confer protection for the muted α-sarcoglycan while minimizing the cytotoxic effects of bortezomib. In the study, the researchers performed an ADMET (absorption, distribution, metabolism, elimination and toxicity) analysis via an artificial intelligence developed by Kantify and trained for assay results prediction on 150,000 compounds. That AI analysis considered 76 ADMET endpoints and ranked the retained molecules in terms of benefit-risk, thus indicating their potential for successful clinical development.
The work by the Évry team led to the identification of 47 possibly-therapeutic compounds. Among them, givinostat in particular showed a strong ability to restore the expression of mutant cell-membrane α-sarcoglycan. Indeed, givinotat is currently in phase III trials for Duchenne muscular dystrophy and showing promising preliminary results. Givinostat appears to inhibit the cell’s autophagic pathway, a system complementary to the proteasome for ensuring the elimination of abnormal proteins.
Thus, beyond the identification of givinostat + bortezomib as a promising combination against LGMD type R3, the I-Stem/Genethon study published in Frontiers in Pharmacology on 27 April 2022, also shed light on the double, autophagic and proteasomic, mechanism involved in the degradation of the misfolded protein. That discovery led the team to extend its study to two diseases with similar protein-breakdown aspects: LGMD type R5 and cystic fibrosis, on which the givinostat + bortezomib combination also showed positive effects.